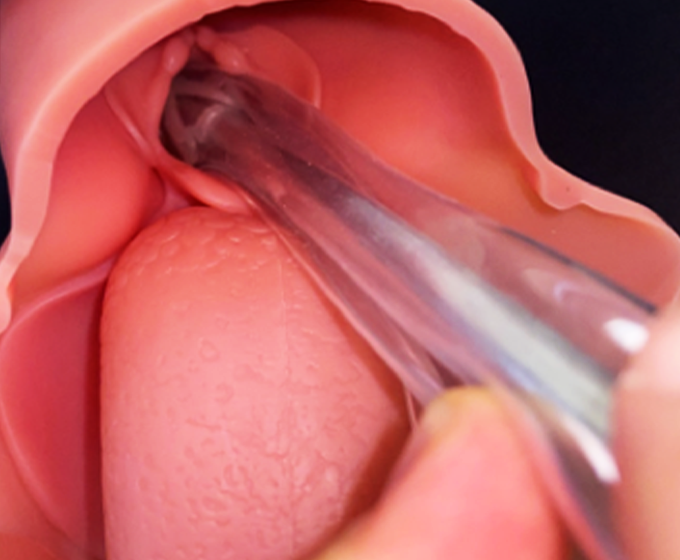
MAY 21, 2020 — UTSA mechanical engineering researchers have developed a new breathing tube designed to solve the problem of instability and tissue damage from the long-term ventilation of COVID-19 patients and emergency medicine.
COVID-19 can spread swiftly in the population and progress rapidly in individual patients. In turn, according to news reports, a patient may require emergency intubation and mechanical ventilation to survive. In order to place traditional ventilator tubes into the throat, doctors and nurses treating patients come in close contact with aerosolized SARS-CoV-2, often with inadequate personal protective equipment.
A critical COVID-19 patient may experience prolonged ventilation, which, according to the Mayo Clinic, can lead to tracheal stenosis, tissue scarring, dislocation and stricture of the arytenoid cartilages. These injuries are more likely to occur if an oversize endotracheal tube or over-pressurized cuff is used or left in position for longer than a week.
The 3D-printed device may reduce medical workers’ exposure to viruses and mitigate physical side effect problems for the patients.
The potential benefits of the new breathing tube are threefold, David Restrepo , explained assistant professor in UTSA’s Department of Mechanical Engineering, who is also one of the creators, “by limiting the exposure of health care providers to patient pathogens through streamlining the intubation process, reducing downstream complications (trachea injuries) and eliminating the need of multiple sizes.”
Other creators of this new breathing tube include R. Lyle Hood , assistant professor in UTSA’s Department of Mechanical Engineering, and military science student David Berard .
The project is a collaboration with UT Health San Antonio and the U.S. Army Institute of Surgical Research. The team filed a provisional application with the U.S. Patent and Trademark Office, which locks in the date of the invention. Once filed, the engineers have up to a year to submit a full application to the PTO for review.
“In parallel with the patent, we are free to develop technology toward clinical use,” Hood said. “We are looking to deploy our technology in animal models this year and human clinical trials for the Food and Drug Administration next year.”